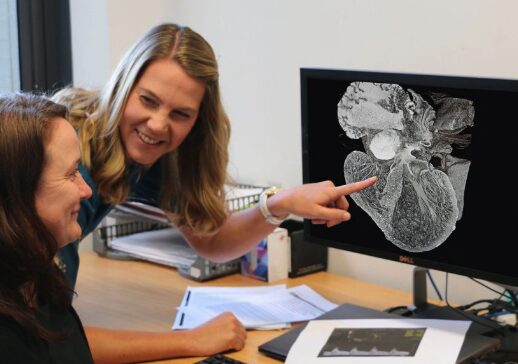
A University of Auckland team creating virtual models of the heart and placenta to study links between low birth weight babies and heart disease has secured $1 million in funding.
For more than a decade, researchers at the Auckland Bioengineering Institute (ABI) at Waipapa Taumata Rau, University of Auckland have been researching the placenta, the organ responsible for feeding babies before they are born.
Around 1-in-10 pregnancies is impacted by fetal growth restriction, where a baby does not grow as well as it should prior to birth. In up to half of these pregnancies, the restricted development is not discovered until the baby is born.
ABI Associate Professors Alys Clark and Jo James are leading a team of scientists investigating the health of the placenta.
“It has become more and more obvious we can’t treat individual organs in isolation,” Assoc Prof Clark says.
“We need to understand more about how the placenta and fetus interact as a whole system.”
Typically, small babies have smaller placentas feeding them before birth, with less blood vessel branching within them, which impacts growth. In addition, at birth these babies have subtle changes in their hearts.
A Marsden grant boost of nearly $1 million over three years will help the team explore how the heart develops differently in pregnancies where fetal growth is impaired. They hope to identify how mechanical links between the placenta and the rest of the fetal cardiovascular system in the womb may impact the baby’s long term heart health.
“It is a stage of rapid development in the baby and the placenta that is challenging to observe with low-resolution clinical imaging tools,” says Assoc Prof Clark.
“So tests are not possible in real pregnancies.”
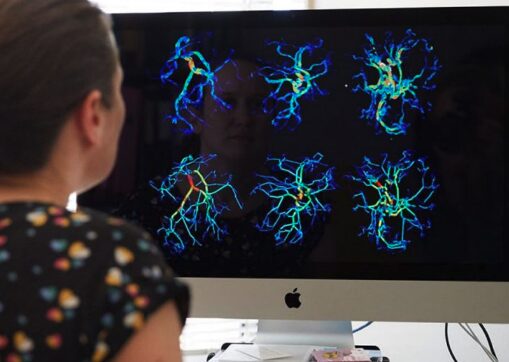
Instead, the team members are using experimental data and novel imaging methods to inform computer-based descriptions of the fetal cardiovascular system. The goal is to help understand which parts of this system contribute the most to the interaction between heart and placenta in pregnancy.
Establishing biomechanical links is an essential step in finding and improving metrics that can predict later cardiovascular dysfunction, and may help medical professionals intervene earlier to reduce the burden of heart disease, Clark says.
While there is evidence of shared mechanisms, both genetic and environmental, in heart and placenta development, the mechanical interactions can only be investigated with this virtual pregnancy approach, she says.
“If we can predict risk of cardiovascular disease early, there is strong evidence that we can do a better job of follow-up tests and monitoring that can improve heart health later in life.”
“With this new knowledge we can start to improve how we detect and manage these pregnancies.”
Auckland Bioengineeing Institute Director, Professor Merryn Tawhai calls the Clark-James research partnership “a powerhouse collaboration doing innovative, world-leading research in pregnancy health”.
She says the project will impact positively on many future lives.
“Marsden funding is highly competitive, so this is a real testament to the novelty and importance of their research.”
Article written by Megan Fowlie.